Apply for SME status today!
ADRES EU, the European subsidiary of ADRES, can provide your company with valuable scientific and regulatory assistance through a simple, quick, and inexpensive process!
Between 2016-2020:
- The success rate for SME marketing authorization applications for human medicines more than doubled, reaching 89% in 2020
- More than 4 in 10 medicines selected for EMA’s PRIME: priority medicines scheme were from SMEs
- SMEs developed nearly 20% of all human medicines recommended for authorization in 2020; half of these target a rare disease.
The European Medicines Agency (EMA) offers different incentives for micro, Small and Medium-sized Enterprises (SMEs). SMEs are eligible for consideration in EU expedite-development programs, as well as to receive regulatory, financial, and administrative assistance supporting the product development process, including:
- Regulatory, administrative, and procedural assistance including SME briefing meetings
- Fee reductions for scientific advice, scientific services, and inspections
- Fee exemptions for certain EMA administrative services
- SMEs can apply for a PRIME on the basis of compelling pre-clinical data and tolerability data from initial clinical trials instead of clinically meaningful improvement of efficacy
- Certification procedure at any time during the development of an ATMP
- Approaching the Innovation task force, which provides a platform to open an informal dialogue with the Agency and proactively identify scientific, legal and regulatory issues arising from their developments
- Deferral of the fee payable for an application for marketing authorization and related activities (e.g., inspections, translations of the product information)
- Fee reductions and exemptions for post-authorization procedures and pharmacovigilance activities
- and more…
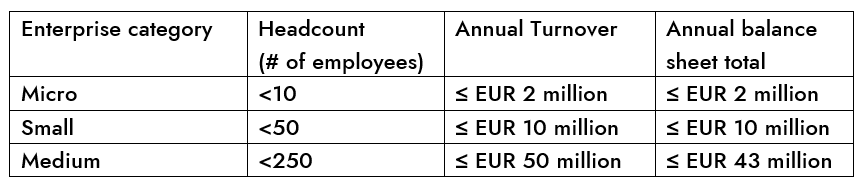
SMEs are enterprises that meet the following criteria:
- Employ fewer than 250 persons and
- Have an annual turnover not exceeding EUR 50 million, and/or an annual balance sheet total not exceeding EUR 43 million.
So, what do you need to do? Almost nothing!
Step 1. Write us to: tanya@adres.bio
Step 2. We will assess your organization’s eligibility for SME status
Step 3. If eligible, we will request an SME status for your organization (to be annexed to ADRES EU).
It takes approximately one month to obtain an SME status, while no EMA fee is required.
https://www.youtube.com/watch?v=Zp-zMrzuLOs[LIY1] This is an explanation of SME status by the EMA